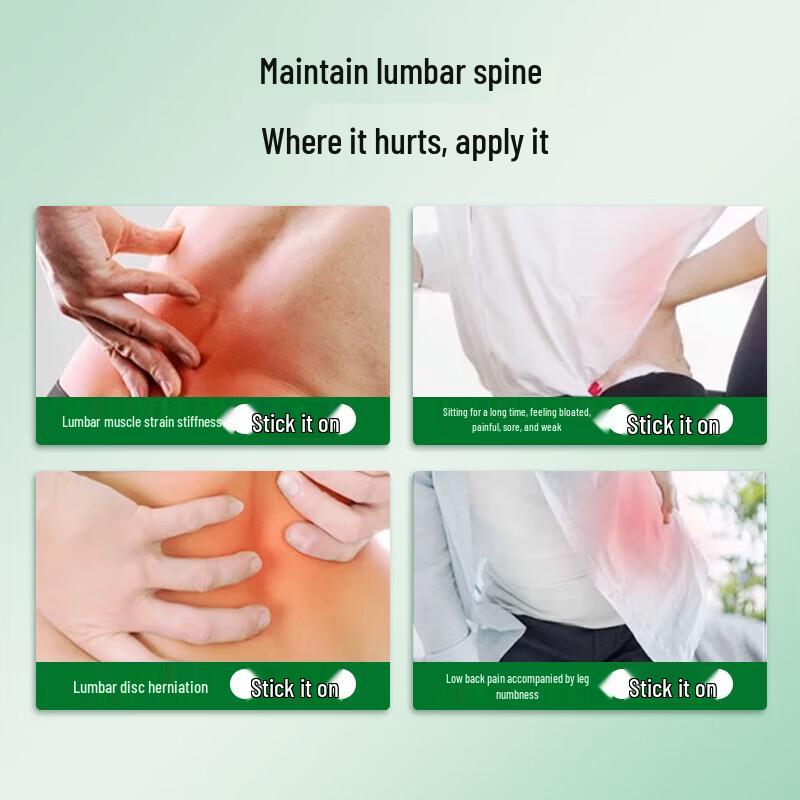
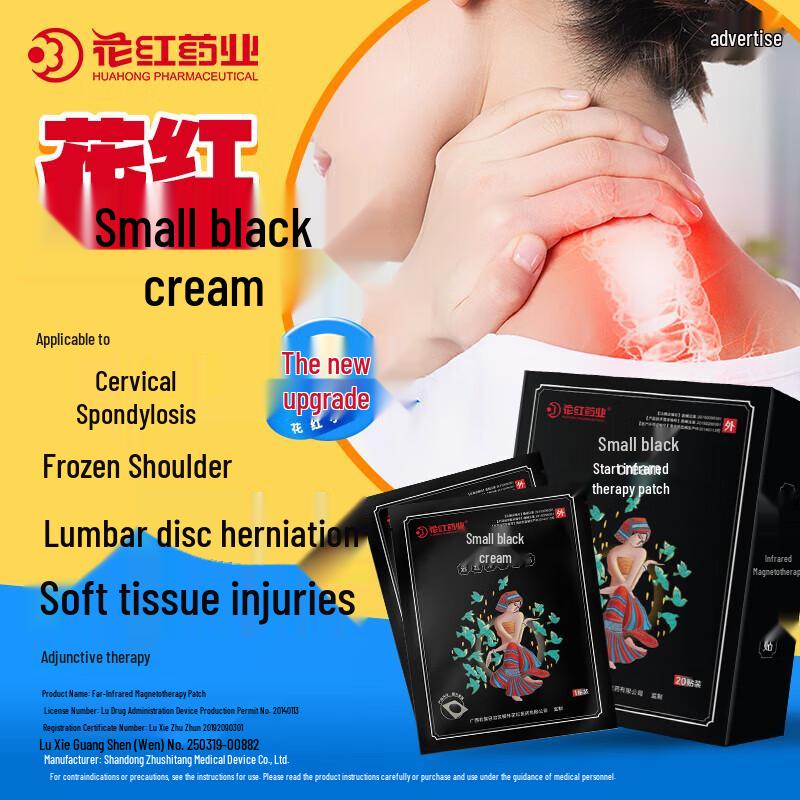
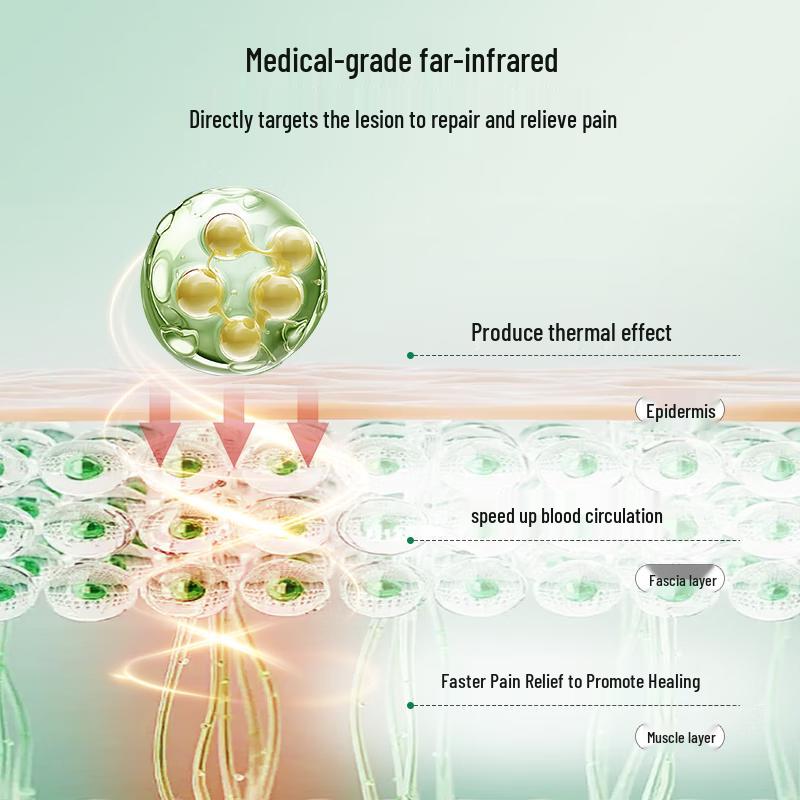
The Hua Hong Far-Infrared Magnetic Therapy Patch is a Class II medical device designed for auxiliary treatment of various musculoskeletal conditions. It utilizes far-infrared ceramic powder and magnetic therapy to help relieve discomfort associated with cervical spondylosis, shoulder periarthritis, hyperosteogeny, lumbar disc herniation, arthritis, muscle strain, and soft tissue injuries. Each patch measures 120mm x 150mm and is recommended for replacement every 24 hours after application to the affected area.
Registrant or filer information: Shandong Zhu's Tang Medical Device Co., Ltd.
model: ZS-Ⅰ120mm×150mm
Contraindications: (1) local metal foreign body; (2) local pacemaker and its vicinity; (3) patients with severe heart, liver, lung, and kidney failure; (4) patients with bleeding and bleeding tendency; (5) lower abdomen of pregnant women; (6) those with obvious adverse reactions to magnetotherapy; (7) those with extremely weak constitution
Specification: 120mm×150mm
Is it a medical device: yes
Structure and composition: This product is made of far-infrared ceramic powder, magnetic sheets, polymer gel, non-woven fabric, and silicone oil paper.
Scope of application: ZS-|Suitable for the auxiliary treatment of cervical spondylosis, periarthritis of the shoulder, osteoproliferation, lumbar disc herniation, arthritis, fibrositis, soft tissue injury, acute lumbar muscle strain.
Production license or filing certificate number: Lu YJX Production Permit 20140113
Storage conditions: This product should be stored in an environment with relative humidity not exceeding 80%, free of corrosive gases, protected from sunlight, dry, well-ventilated, and clean.
Domestic/Imported: Domestic
category: General
Safety Instructions: Clean the affected area, confirm the packaging is intact, open the package, take out the far-infrared magnetotherapy patch, remove the release liner of the patch, apply it to the affected area, and replace it every 24 hours.
Units of measurement: box
Is the inventory managed by lot number?: yes
Medical Device Registration Certificate Number or Filing Certificate Number: Lu械注准20192090301
Product Technical Requirement Number: Lu械注准20192090301
manufacturer: Shandong Zhu's Tang Medical Device Co., Ltd.
Medical device name: Far-infrared magnetic therapy patch
Medical device category: Class II 09